IOL Chemicals and Pharmaceuticals Reports Q3 FY23 Results
7th February 2023, New Delhi:
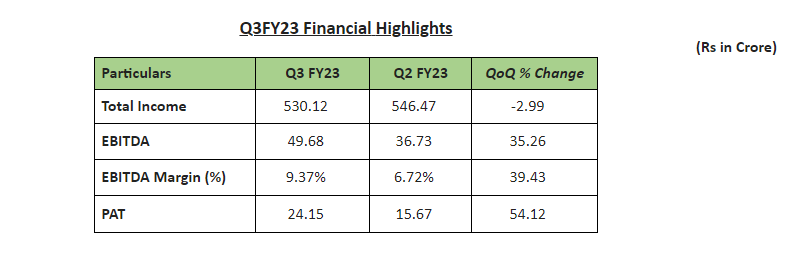
IOL Chemicals and Pharmaceuticals Limited, a leading manufacturer of pharmaceutical (APIs) and specialty chemicals reported results for the 3rd quarter and nine months ended 31st December 2023 as highlighted herein below:
Operational highlights
- Granted a patent for “an improved safe process for the preparation of Sartan drugs of Formula I” by Indian Patent Office
- Recently received EUGMP Certification from National Institute of Pharmacy and Nutrition, Hungary, which will help the Company in more penetration in European market for its products.
Commenting on the quarterly performance, Dr Sanjay Chaturvedi, Executive Director & CEO said, “The quarterly performance reflects our commitment to control our costs and improve our margin trajectory. For the third quarter, EBITDA and Net Profit margins – both have improved sequentially. Apart from cost rationalisation, we have completed our capacity enhancement of Paracetamol by 1,800 MTPA to take total capacity to 3,600 MTPA with Backward integration of Para Amino Phenol (PAP) and we expect this capacity enhancement to boost volume growth from next quarter onwards.”
About IOL Chemicals and Pharmaceuticals Limited (IOLCP)
Company was established in 1986, listed on National Stock Exchange of India Ltd (Code: IOLCP) and BSE Ltd (Code: 524164) is one of the leading pharmaceutical (APIs) Company and is significant player in the specialty chemicals space with world class facilities. IOLCP has wide presence across major therapeutic categories like, Pain Management, anti-convulsants, antidiabetes, anti- cholesterol and anti-platelets.
The Company’s product portfolio includes APIs; Ibuprofen, Metformin, Fenofibrate, Clopidogrel, Lamotrigine, Pantoprazole, Paracetamol and specialty chemicals such as Ethyl Acetate, Iso Butyl Benzene, Mono Chloro Acetic Acid and Acetyl Chloride.
Company is World’s largest producer of the Ibuprofen with an installed capacity of 12,000 TPA and having backward integrated manufacturing facility. The Company has DSIR approved R&D which is fully equipped to validate existing processes.
The Central and State Government approved Effluent Treatment Plant (ETP) had been set up with Zero Liquid Discharge (ZLD) system along with 17 MW captive Co-Generation plant for self -reliance. The Company is ISO 9001:2015, 14001:2015 and BS OHSAS 18001:2007 certified.
IOLCP’s overseas customers are spread out across several countries including UK, Belgium, Hungry, Spain, Germany, Italy, Netherlands, Switzerland, Poland, Ireland, USA, Peru, Brazil, Argentina, Colombia, Indonesia, South Korea, Thailand, Bangladesh, Turkey, U.A.E., China, Hong Kong, Egypt etc. Products are sold primarily to Branded Generic formulators both in India & Overseas.
